
U.S. SPECT PRODUCTS | OTHERS
Ioflupane I 123 Injection
This information is intended for U.S. healthcare professionals only.
Product information
Ioflupane I 123 Injection (a generic for DaTscanTM): Broad accessibility every weekday helps meet the growing demand1 and enables patients to make the most of their mornings
- Broader accessibility helps meet the growing demand for testing1
- Deliveries Monday through Friday by 7 AM to most US locations enable patients to be scanned earlier and potentially more scans to be scheduled per day
- Competitive pricing and negotiable contract terms available
- The same reimbursement codes as DaTscan are used (HCPCS code A9584 and CPT® codes 78803 and 78830)
Indication & Usage
Ioflupane I 123 is indicated as an adjunct to other diagnostic evaluations for striatal dopamine transporter visualization using single photon emission computed tomography (SPECT) brain imaging in adult patients with:
- suspected Parkinsonian syndromes (PS) or
- suspected dementia with Lewy bodies (DLB)
Important risk information
CONTRAINDICATIONS
- Ioflupane I 123 Injection is contraindicated in patients with known serious hypersensitivity to ioflupane I 123.
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Hypersensitivity reactions, including dyspnea, edema, rash, erythema, and pruritus, have been reported following Ioflupane I 123 Injection administration.
- Thyroid Accumulation: The Ioflupane I 123 Injection may contain up to 6% of free iodide (iodine-123). Thyroid uptake of iodine-123 may result in an increased long-term risk for thyroid neoplasia. To decrease thyroid accumulation of I-123, block the thyroid gland before administration of Ioflupane I 123 Injection.
- Radiation Risk: Ioflupane I 123 Injection emits radiation and must be handled with safety measures to minimize radiation exposure to clinical personnel and patients.
ADVERSE REACTIONS
- In clinical trials, headache, nausea, vertigo, dry mouth, or dizziness of mild to moderate severity were reported. In postmarketing experience, hypersensitivity reactions and injection-site pain have been reported.
DRUG INTERACTIONS
- Drugs that bind to the dopamine transporter with high affinity may interfere with the Ioflupane I 123 Injection image. The impact of dopamine agonists and antagonists on Ioflupane I 123 Injection imaging results has not been established.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Radioactive iodine products cross the placenta and can permanently impair fetal thyroid function. Administration of a thyroid blocking agent is recommended before the use of Ioflupane I 123 Injection in a pregnant woman. All radiopharmaceuticals have potential to cause fetal harm. There are no available data on Ioflupane I 123 Injection use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Advise pregnant women of the potential risks of fetal exposure to radiation with the administration of Ioflupane I 123 Injection.
- Lactation: Iodine 123 (I 123), the radionuclide in Ioflupane I 123 Injection, is present in human milk. There is no information on the effects on breastfed infants or on milk. Advise a lactating woman to interrupt breastfeeding and pump and discard breast milk for at least 6 days after Ioflupane I 123 Injection administration to minimize radiation exposure to a breastfeeding infant.
- Pediatric Use: The safety and efficacy of Ioflupane I 123 Injection have not been established in pediatric patients.
- Geriatric Use: There were no differences in responses between elderly patients and younger patients that would require a dose adjustment observed in the Parkinsonian syndrome studies.
- Renal Impairment: Ioflupane I 123 Injection is excreted by the kidney and patients with severe renal impairment may have increased radiation exposure and altered Ioflupane I 123 Injection images.
OVERDOSAGE
- The risks of overdose relate predominantly to increased radiation exposure, with the long-term risk for neoplasia. In case of overdosage of radioactivity, frequent urination and defecation should be encouraged to minimize radiation exposure to the patient.
Prior to Ioflupane I 123 Injection administration, please read the full Prescribing Information for additional Important Risk Information.
Frequently asked questions
Yes. Ioflupane I 123 Injection now has 2 indications and is substitutable for DaTscan.2,3
A generic drug is a medication created to be the same as an existing approved brand-name drug in dosage form, safety, strength, route of administration, quality, and performance characteristics.
Generic medicines work the same as brand-name medicines.
The recommended dose is 111 MBq to 185 MBq (3 mCi to 5 mCi) administered intravenously over at least 20 seconds. The administered dose is exactly the same for both indications.2
To place an order, contact your Curium representative or call customer service at 888-744-1414, Monday through Friday, 7 AM to 5 PM CT.
Orders should be placed by Friday at 6 PM ET for delivery Monday through Wednesday by 7 AM or by Monday at 6 PM ET for delivery by Thursday or Friday at 7 AM.
If you order by Friday at 6 PM ET, you can expect delivery by 7 AM Monday through Wednesday. If you order by Monday at 6 PM ET, you can expect delivery by 7 AM Thursday or Friday.
If you receive your dose through a nuclear pharmacy, please contact them about delivery time.
HCPCS code A9584 and CPT® codes 78803 and 78830 should be used.
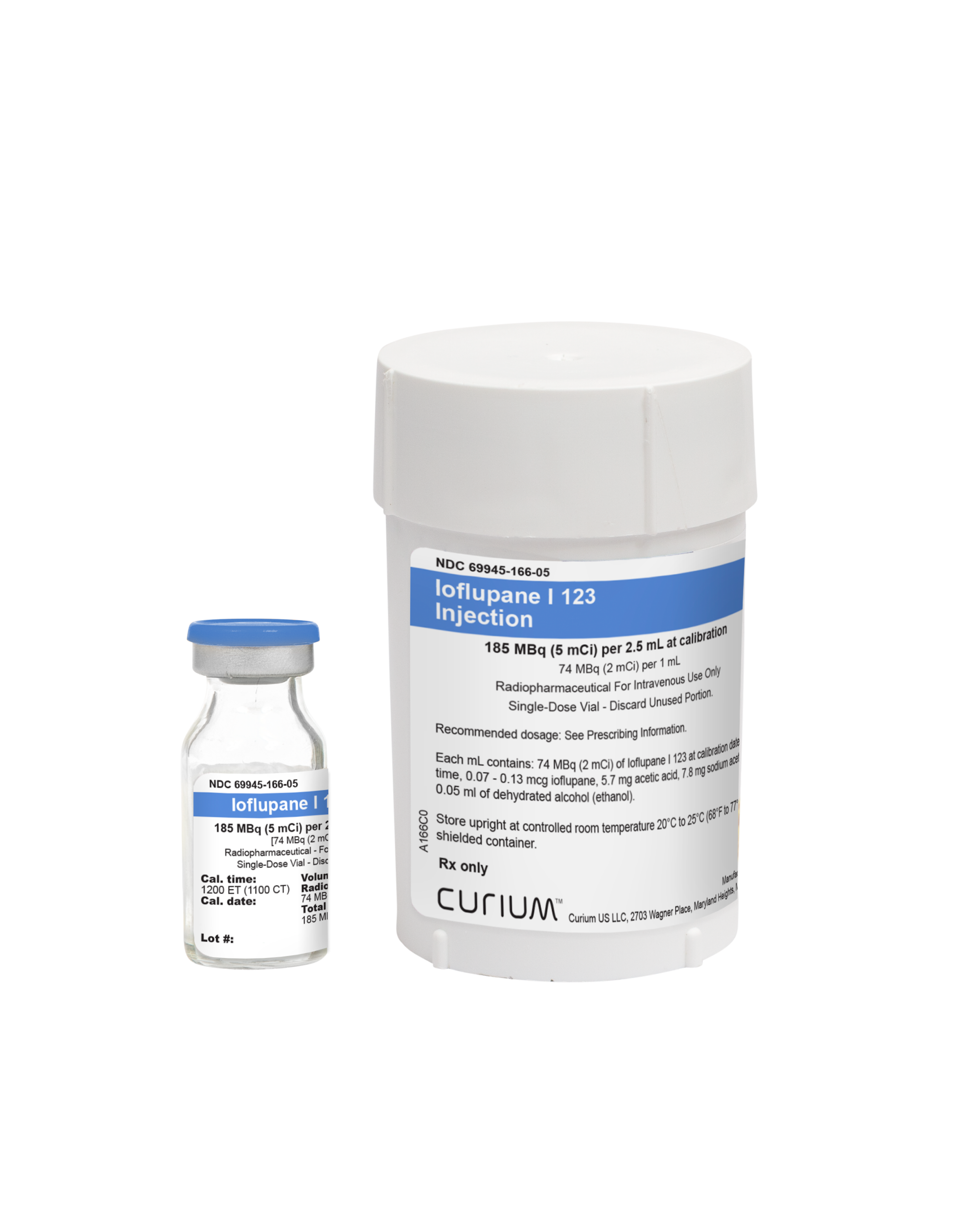
Ioflupane I 123 Injection is a sterile, clear, colorless solution supplied in a 10 mL single-dose vial containing a total volume of 2.5 mL with a total radioactivity of 185 MBq (5 mCi) of Ioflupane I 123 at calibration time (12 PM ET) and date. Each mL contains 74 MBq (2 mCi) of Ioflupane I
123 at calibration time and date. Each vial is enclosed in a lead container of appropriate thickness for radiation protection.2
How is this product supplied
Order information
| Description | Qty | Unit | Size | Order # | NDC |
|---|---|---|---|---|---|
| Ioflupane I 123 Injection 10-mL vial | 1 | Vial | 10 mL | N/A | 69945-166-05 |
To place an order, contact your Curium representative or call customer service at 888-744-1414, Monday through Friday, 7 AM to 5 PM CT.
References
1 Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21. doi:10.1038/s41531-018-0058-0.
2 Ioflupane I 123 Injection. Package insert. Curium US LLC; December 2022.
3 DaTscan. Package insert. GE Healthcare; November 2022.
DaTscan™ is a trademark of GE Healthcare Limited.
CPT® is a registered trademark of the American Medical Association.
© 2024 Curium US LLC.
IO0015 0124