
U.S. SPECT PRODUCTS | OTHERS
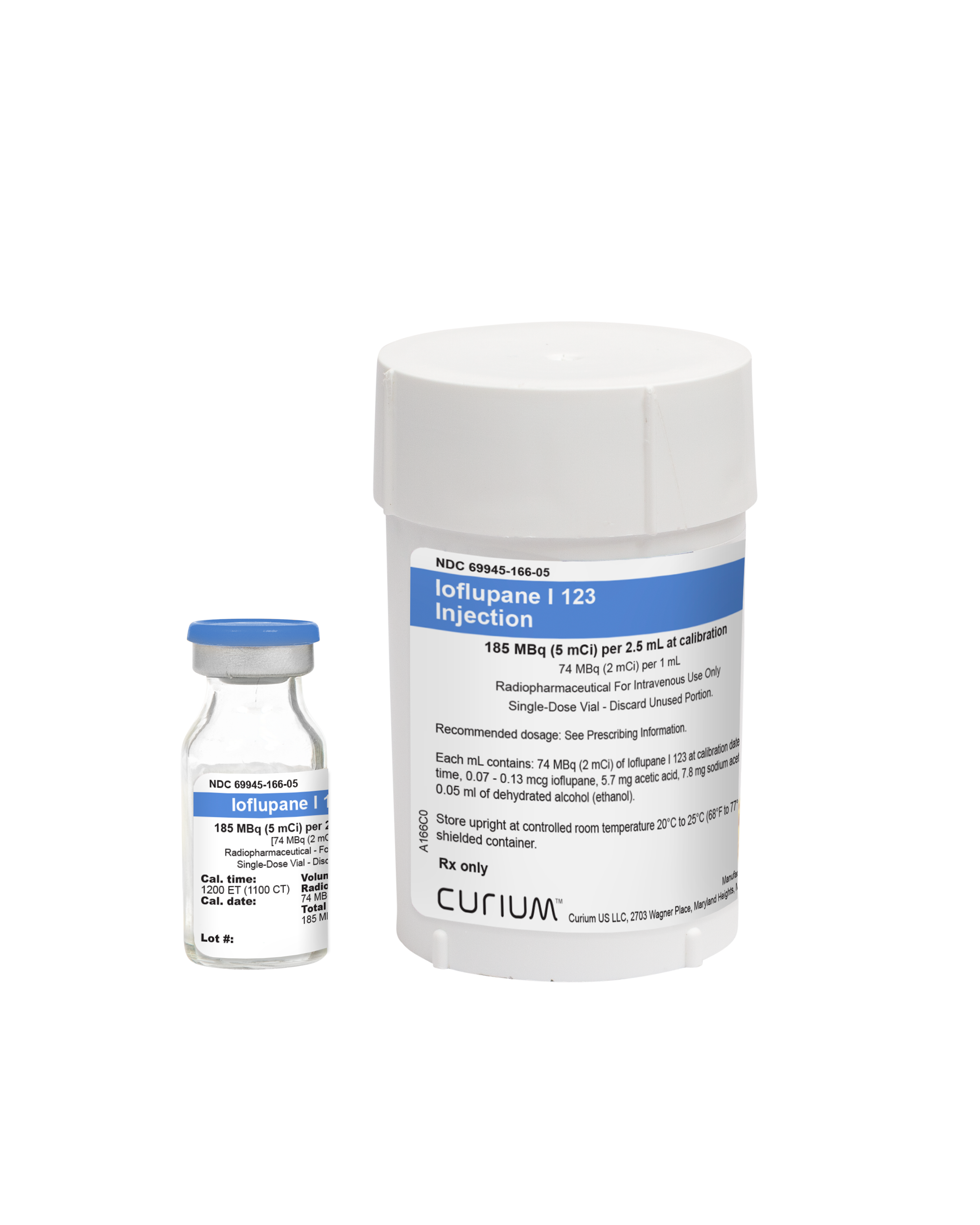
Ioflupane I 123 Injection
Diese Website richtet sich an medizinische Fachkreise innerhalb der Vereinigte Staaten.
Produktinformation
Ioflupane I 123 Injection (a generic for DaTscan™) will enable more patients to access the scans they need so they can get a timely diagnosis
- Broader accessibility helps meet the growing demand for testing1
- Deliveries Monday through Thursday by 7 AM to most US locations enable patients to be scanned earlier and potentially more scans to be scheduled per day
- Competitive pricing and negotiable contract terms available
- The same reimbursement codes as DaTscan are used (HCPCS code A9584 and CPT® codes 78803 and 78830)
Indications and Usage
Ioflupane I 123 Injection is a radiopharmaceutical indicated for striatal dopamine transporter visualization using single-photon emission computed tomography (SPECT) brain imaging to assist in the evaluation of adult patients with suspected parkinsonian syndromes (PSs). In these patients, Ioflupane I 123 Injection may be used to help differentiate essential tremor from tremor due to PS (idiopathic Parkinson’s disease [PD], multiple system atrophy [MSA], and progressive supranuclear palsy [PSP]). Ioflupane I 123 Injection is an adjunct to other diagnostic evaluations.
Ioflupane I 123 Injection was not designed to distinguish among PD, MSA, and PSP. The effectiveness of Ioflupane I 123 Injection as a screening or confirmatory test and for monitoring disease progression or response to therapy has not been established.
CONTRAINDICATIONS
- Ioflupane I 123 Injection is contraindicated in patients with known hypersensitivity to the active substance, any of the excipients, or iodine
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Hypersensitivity reactions, generally consisting of skin erythema and pruritus, have been reported following Ioflupane I 123 Injection administration
- Thyroid Accumulation: The Ioflupane I 123 Injection may contain up to 6% of free iodide (iodine 123 or I-123). To decrease thyroid accumulation of I-123, block the thyroid gland at least one hour before administration of Ioflupane I 123 Injection; failure to do so may increase the long-term risk for thyroid neoplasia
ADVERSE REACTIONS
- In clinical trials, headache, nausea, vertigo, dry mouth, or dizziness of mild to moderate severity were reported. In postmarketing experience, hypersensitivity reactions and injection-site pain have been reported
DRUG INTERACTIONS
- Drugs that bind to the dopamine transporter with high affinity may interfere with the Ioflupane I 123 Injection image. The impact of dopamine agonists and antagonists on Ioflupane I 123 Injection imaging results has not been established
- These potentially interfering drugs consist of: amoxapine, amphetamine, benztropine, bupropion, buspirone, cocaine, mazindol, methamphetamine, methylphenidate, norephedrine, phentermine, phenylpropanolamine, selegiline, and sertraline. Selective serotonin reuptake inhibitors (paroxetine and citalopram) may increase or decrease ioflupane binding to the dopamine transporter. Whether discontinuation of these drugs prior to Ioflupane I 123 Injection administration may minimize the interference with an Ioflupane I 123 Injection image is unknown
USE IN SPECIFIC POPULATIONS
- Pregnancy: Radioactive iodine products cross the placenta and can permanently impair fetal thyroid function. Administration of a thyroid blocking agent is recommended before the use of Ioflupane I 123 Injection in a pregnant woman. All radiopharmaceuticals have potential to cause fetal harm. There are no available data on Ioflupane I 123 Injection use in pregnant woman to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Advise pregnant women of the potential risks of fetal exposure to radiation with the administration of Ioflupane I 123 Injection
- Lactation: Iodine 123 (I 123), the radionuclide in Ioflupane I 123 Injection, is present in human milk. There is no information on the effects on breastfed infants or on milk. Advise a lactating woman to interrupt breastfeeding and pump and discard breast milk for at least 6 days after Ioflupane I 123 Injection administration to minimize radiation exposure to a breastfeeding infant
- Pediatric Use: The safety and efficacy of Ioflupane I 123 Injection have not been established in pediatric patients
- Geriatric Use: There were no differences in responses between elderly patients and younger patients that would require a dose adjustment
- Renal and Hepatic Impairment: The effect of renal or hepatic impairment on Ioflupane I 123 Injection imaging has not been established. The kidney excretes Ioflupane I 123 Injection; patients with severe renal impairment may have increased radiation exposure and altered Ioflupane I 123 Injection image
OVERDOSAGE
- It is unknown whether or not ioflupane is dialyzable. The major risks of overdosage relate to increased radiation exposure and long-term risk for neoplasia. In case of radioactivity overdosage, frequent urination and defecation should be encouraged to minimize radiation exposure to the patient
PROCEDURE — Radiation Safety
- Ioflupane I 123 Injection emits radiation and must be handled with safety measures to minimize radiation exposure to clinical personnel and patients
Prior to Ioflupane I 123 Injection administration, please read the full Prescribing Information for additional Important Risk Information.
Frequently asked questions
Yes. Ioflupane I 123 Injection is substitutable for DaTscan.2,3
A generic drug is a medication created to be the same as an existing approved brand-name drug in dosage form, safety, strength, route of administration, quality, and performance characteristics.
Generic medicines work the same as brand-name medicines.
The recommended dose is 111 MBq to 185 MBq (3 mCi to 5 mCi) administered intravenously.2
To place an order, contact your Curium representative or call customer service at 888-744-1414, Monday through Friday, 7 AM to 5 PM CT.
Orders should be placed by Friday at 6 PM ET for delivery Monday through Wednesday by 7 AM or by Monday at 6 PM ET for delivery by Thursday at 7 AM.
If you order by Friday at 6 PM ET, you can expect delivery by 7 AM Monday through Wednesday. If you order by Monday at 6 PM ET, you can expect delivery by 7 AM Thursday.
If you receive your dose through a nuclear pharmacy, please contact them about time of delivery.
HCPCS code A9584 and CPT® codes 78803 and 78830 should be used.
Ioflupane I 123 Injection is a sterile, clear, colorless solution supplied in a 10 mL single-dose vial containing a total volume of 2.5 mL with a total radioactivity of 185 MBq (5 mCi) of Ioflupane I 123 at calibration time (12PM ET) and date. Each mL contains 74 MBq (2 mCi) of Ioflupane I 123 at calibration time and date. Each vial is enclosed in a lead container of appropriate thickness for radiation protection.2
How is this product supplied
Order information
| Description | Qty | Unit | Size | Order # | NDC |
|---|---|---|---|---|---|
| Ioflupane I 123 Injection 10-mL vial | 1 | Vial | 10 mL | N/A | 69945-166-05 |
To place an order, contact your Curium representative or call customer service at 888-744-1414, Monday through Friday, 7 AM to 5 PM CT.
References
1 Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21. doi:10.1038/s41531-018-0058-0.
2 Ioflupane I 123 Injection. Package insert. Curium US LLC; December 2021.
3 DaTscan Package insert. GE Healthcare; March 2020.
DaTscan™ is a trademark of GE Healthcare Limited.
CPT® is a registered trademark of the American Medical Association.