
U.S. Spect products | Lung
Xenon Xe 133 Gas
This information is intended for U.S. healthcare professionals only.
Product information
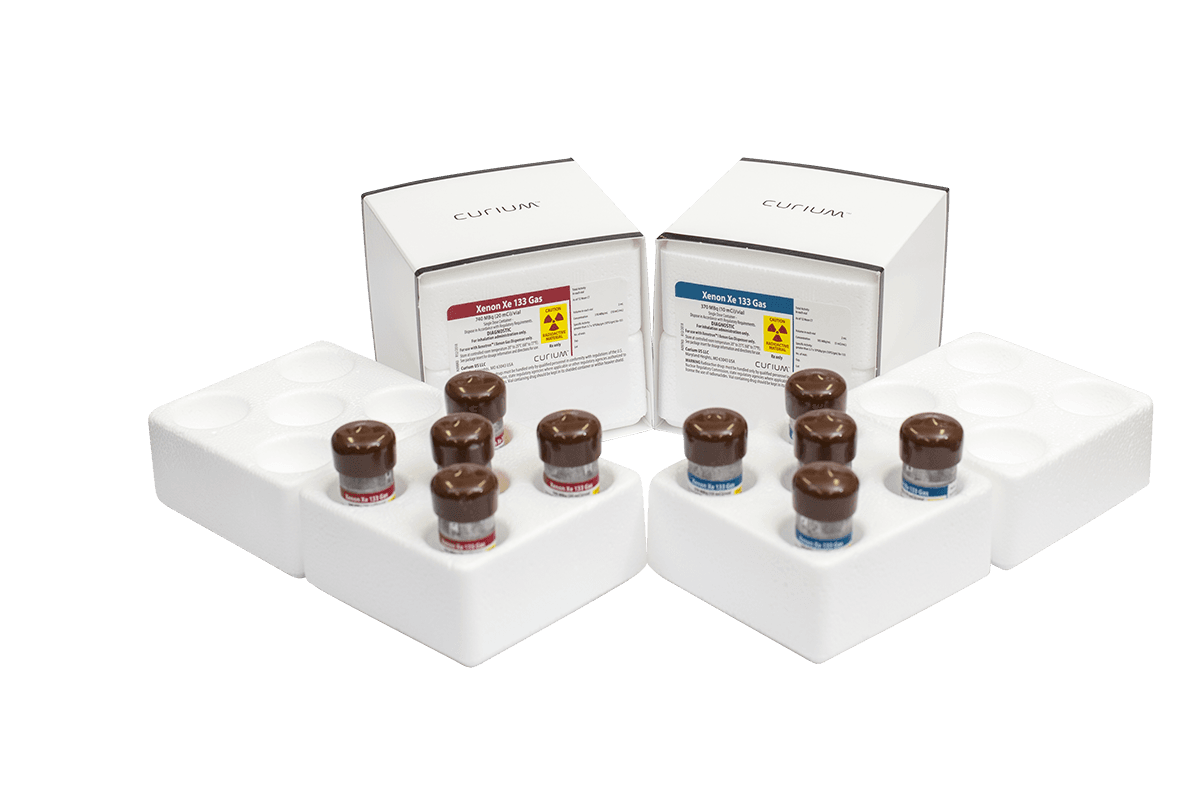
Convenient single use vial
Each single use vial is encased in its own individually-labeled lead safe to provide reliable radiation protection. Packaging is offered in one, three and five vial combinations for ease of distribution to accommodate facilities of all sizes.
Early morning delivery
Our vast logistics network allows for, in many cases, delivery of Xenon Xe 133 Gas prior to 7:00 a.m.
Reliable supply
Our vertically integrated radiopharmaceutical manufacturing network is the largest in the industry, providing significant stability and reliability of supply.
Not made with natural rubber latex
Xenon Xe 133 Gas components are not made with natural rubber latex.
Manufactured from 100% LEU
Curium is committed to eliminating the use of nuclear weapons-grade uranium in the production of our medical isotopes.
Indication & Usage
Xenon Xe 133 Gas has been shown to be valuable for diagnostic inhalation studies for the evaluation of pulmonary function, for imaging the lungs and may also be applied to assessment of cerebral blood flow.
Important risk information
Warnings and precautions
- Xenon Xe 133 Gas delivery systems, i.e., respirators or spirometers, and associated tubing assemblies must be leakproof to avoid loss of radioactivity into the laboratory environs not specifically protected by exhaust systems.
- Xenon Xe 133 Gas adheres to some plastics and rubber and should not be allowed to stand in tubing or respirator containers. Loss of radioactivity due to such adherence may render the study nondiagnostic.
- Xenon Xe 133 Gas as well as other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Also, care should be taken to minimize radiation exposure to the patients consistent with proper patient management.
- Exhaled Xenon Xe 133 Gas should be controlled in a manner that is in compliance with the appropriate regulations of the government agency authorized to license the use of radionuclides.
- Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides.
- Transfer the appropriate Xenon Xe 133 Gas dose from the Xenon Xe 133 Gas unit dose vial(s) to a breathing device or spirometer utilizing the Xenotron™ I Xenon Gas Dispenser. Follow the directions for use that are provided with the Xenotron I Xenon Gas Dispenser.
Adverse reactions
- Adverse reactions specifically attributable to Xenon Xe 133 Gas have not been reported.
Use in specific populations
- Pregnancy Category C. Xenon Xe 133 Gas should be given to a pregnant woman only if clearly needed.
- Breastfeeding: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Xenon Xe 133 Gas is administered to a nursing woman.
- Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Source will provide the following activity of day of receipt at 12:00 Noon (CST). View decay chart
Frequently asked questions
Xenon Xe 133 Gas can be ordered through your local Sales Representative, or our Customer Service Department at 888.744.1414, option 1 then option 2. Orders are accepted Monday – Friday, 7:00 am to 5:00 pm. CST.
Call Curium Pharmacovigilance / Product Monitoring Department at 866.789.2211
How is this product supplied
Order information
| Description | Qty | Unit | Size | Order # | NDC |
|---|---|---|---|---|---|
| Xenon Xe 133 Gas – 1 Vial | 1 | 1 | 10 mCi | N097K1 | 69945-097-11 |
| Xenon Xe 133 Gas – 3 Vial | 1 | 3 | 10 mCi | N097K3 | 69945-097-13 |
| Xenon Xe 133 Gas – 5 Vial | 1 | 5 | 10 mCi | N097K5 | 69945-097-15 |
| Xenon Xe 133 Gas – 1 Vial | 1 | 1 | 20 mCi | N098U1 | 69945-098-21 |
| Xenon Xe 133 Gas – 3 Vial | 1 | 3 | 20 mCi | N098U3 | 69945-098-23 |
| Xenon Xe 133 Gas – 5 Vial | 1 | 5 | 20 mCi | N098U5 | 69945-098-25 |
References
1 Xenon Xe 133 Gas [package insert]. Maryland Heights, MO: Mallinckrodt Nuclear Medicine LLC; 2016. Based on Xenon Xe 133 Half-life 5.245 Days.