
U.s. Spect products | Others
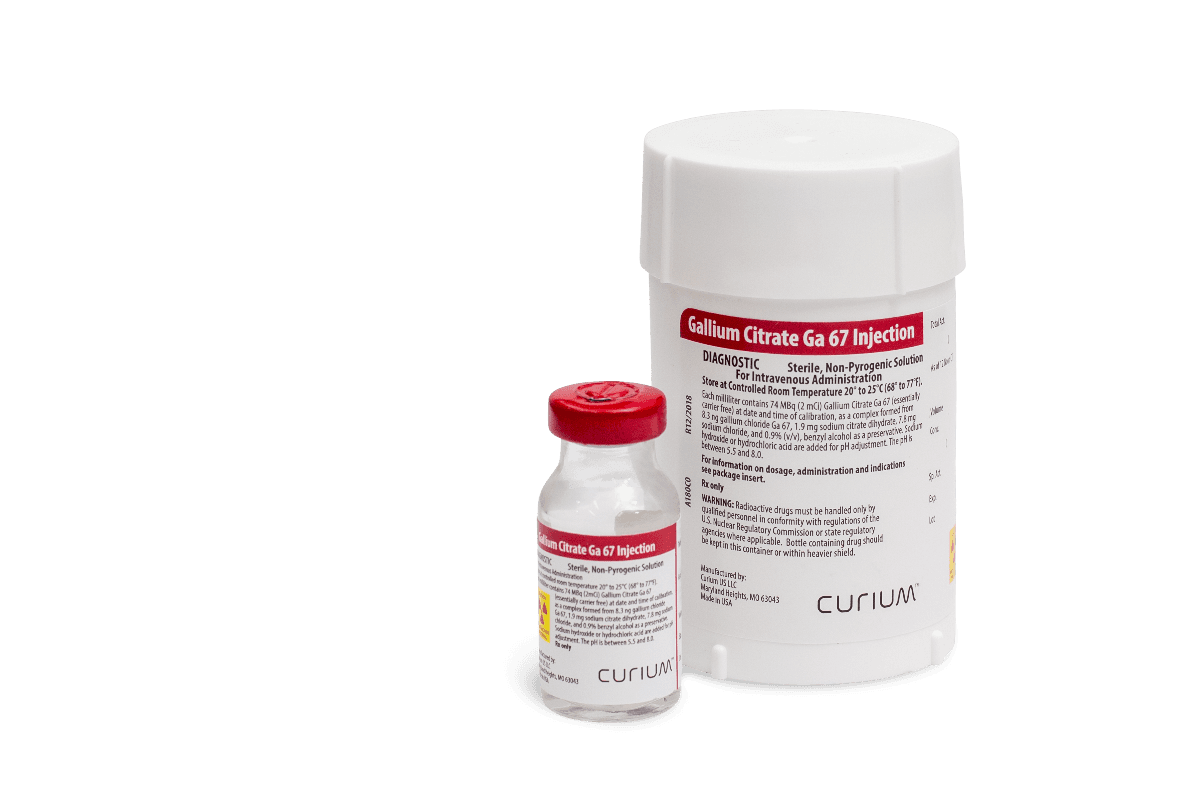
Gallium Citrate Ga 67 Injection
This information is intended for U.S. healthcare professionals only.
Product information
Gallium Citrate Ga 67 Injection is supplied in a 10 milliliter vial as an isotonic, sterile, non-pyrogenic solution. Each milliliter of the isotonic solution contains 74 megabecquerels (2 millicuries) of gallium Ga-67 on the calibration date as a complex formed from 8.3 nanograms gallium chloride Ga-67, 1.9 milligrams of sodium citrate dihydrate, 7.8 milligrams of sodium chloride and 0.9 percent benzyl alcohol (v/v) as a preservative. The pH is adjusted to between 5.5 to 8.0 with hydrochloric acid and/or sodium hydroxide solution.
Gallium Ga-67, with a half-life of 78.26 hours, is cyclotron produced by the proton irradiation of en riched zinc. At the time of calibration the drug contains no more than 0.02% gallium Ga-66 and no more than 0.2% zinc Zn-65. The concentration of each radionuclidic impurity changes with time. At expiration, the drug contains no more than 0.001% gallium Ga-66 and no more than 1.0% zinc Zn-65. No carrier has been added.
Indication & Usage
Gallium Citrate Ga 67 Injection may be useful to demonstrate the presence and extent of Hodgkin’s disease, lymphoma, and bronchogenic carcinoma.
Positive Gallium Citrate Ga 67 Injection uptake in the absence of prior symptoms warrants follow-up as an indication of a potential disease state. Gallium Citrate Ga 67 Injection may be useful as an aid in detecting some acute inflammatory lesions.
Important risk information
Warnings and precautions
- Certain pathologic conditions may yield up to 40 percent false negative Gallium Ga 67 studies. Therefore, a negative study cannot be definitely interpreted as ruling out the presence of disease.
- The use of gallium with lymphocytic lymphoma is not recommended at this time because it does not accumulate Gallium Ga 67 sufficiently for unequivocal imaging.
- Gallium Ga 67 localization cannot differentiate between tumor and acute inflammation and other diagnostic studies must be added to define the underlying pathology.
- Care should be taken to minimize radiation exposure to the patient consistent with proper management and to insure minimum radiation exposure to occupational workers.
- The user should follow the directions carefully and adhere to strict aseptic technique.
- Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides produced by nuclear reactor or particle accelerator and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Adverse reactions
- Rare occurrences of allergic reactions, skin rash and nausea have been reported in association with Gallium Citrate Ga 67 use.
Use in specific populations
- Pregnancy C: should only be given if clearly needed. Not studied in pregnancy.
- Gallium Citrate Ga 67 is known to be excreted in human milk therefore formula feedings should be substituted for breast feedings.
- Safety and efficacy has not yet been established in pediatrics (<18 years of age).
Frequently asked questions
It may demonstrate the presence and extent of Hodgkin’s disease, lymphoma, and bronchogenic carcinoma. However, further studies should be done to determine final diagnosis.
Call Curium Pharmacovigilance / Product Monitoring Department at 866.789.2211
How is this product supplied
Order information
| Description | Qty | Unit | Size | Order # | NDC |
|---|---|---|---|---|---|
| Gallium Citrate Ga 67 Injection – 6 mCi/vial | 1 | Each | 3mL | N180G0 | 69945-180-06 |
| Gallium Citrate Ga 67 Injection – 12 mCi/vial | 1 | Each | 6mL | N180M0 | 69945-180-12 |