
U.S Spect products | Bone
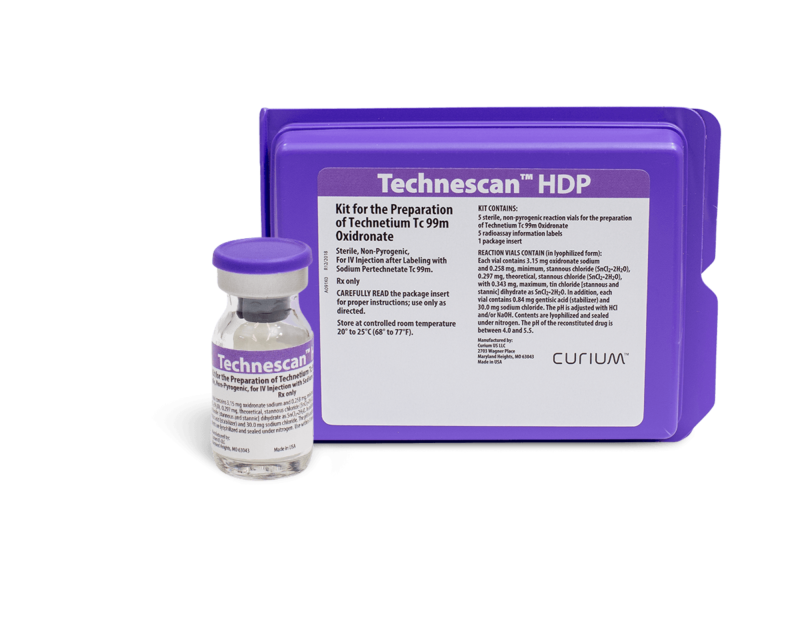
Technescan™ HDP
(kit for the preparation of technetium Tc 99m oxidronate)
This information is intended for U.S. healthcare professionals only.
Product information
Technescan™ HDP is supplied as a lyophilized powder, packaged under nitrogen in vials for intravenous administration after reconstitution with ADDITIVE-FREE sodium pertechnetate Tc 99m. Each vial contains 3.15 mg oxidronate sodium and 0.258 mg, minimum, stannous chloride (SnCl2•2H2O), 0.297 mg, theoretical, stannous chloride (SnCl2•2H2O) with 0.343 mg, maximum, tin chloride [stannous and stannic] dihydrate as SnCl2•2H2O as active ingredients. In addition, each vial contains 0.84 mg gentisic acid as a stabilizer and 30.0 mg sodium chloride. The pH is adjusted with hydrochloric acid and/or sodium hydroxide. The pH of the reconstituted drug is between 4.0 and 5.5. The contents of the vial are sterile and nonpyrogenic
Indication & Usage
Technescan™ HDP (kit for the preparation of technetium Tc 99m oxidronate) is a diagnostic skeletal imaging agent used to demonstrate areas of altered osteogenesis in adult and pediatric patients.
Important risk information
Warnings and precautions
- It is essential that the user follow the directions carefully and adhere to strict aseptic procedures during preparation. Sodium pertechnetate Tc 99m solutions which contain an oxidizing agent or saline solutions containing preservatives are not suitable for use in the preparation of Technescan HDP Tc 99m.
- Contents of the vial are intended only for use in the preparation of Technetium Tc 99m Oxidronate and are NOT to be administered directly to the patient.
- Technetium Tc 99m Oxidronate should be formulated within eight (8) hours prior to clinical use.
- Technetium Tc 99m Oxidronate as well as other radioactive drugs, must be handled with care, and appropriate safety measures should be used to minimize radiation exposure to the patients consistent with proper patient management and to insure minimum radiation exposure to occupational workers.
- Radiopharmaceuticals should be used only by physicians who are qualified by specific training in the safe use and handling of radionuclides
- To help reduce the radiation dose to the bladder, as well as other target organs, the patient should increase his or her fluid intake (unless medically contraindicated) and void as often as possible after the injection of Technetium Tc 99m Oxidronate for six hours after the imaging procedure.
- Technetium Tc 99m Oxidronate may cause life threatening hypersensitivity reactions. Have cardiopulmonary resuscitation equipment and personnel available and monitor all patients for hypersensitivity reactions.
Adverse reactions
- Serious adverse reactions may include hypersensitivity reactions.
- Nausea and vomiting have infrequently been associated with Technetium Tc 99m Oxidronate.
Use in specific populations
- Breast feeding: Should be temporarily discontinued because Technetium Tc 99m is excreted in human milk during lactation.
- Pregnancy: Technescan HDP should not be administered to pregnant women unless it is considered that the benefits to be gained outweigh the potential hazards to the fetus.
Frequently asked questions
300 mCi (volume 3 to 6 mL) – see package insert for additional details
Per the package insert, the recommended pediatric dose is 7.4 MBq (0.2mCi)/kg with a range of 7.4 to 13 MBq (0.2mCi to 0.35mCi)/kg. The recommended minimum total pediatric dose is 37 MBq (1.0mCi). The maximum total dose injected into a pediatric patient is 740 MBq (20.0 mCi). The maximum dose of oxidronate sodium should not exceed 2 mg. See package insert for full prescribing information.
The reconstituted vial must be used within eight (8) hours of preparation.
Technescan HDP can be ordered through your local Sales Representative, or our Customer Service Department at 888.744.1414, option 1 then option 2. Orders are accepted Monday – Friday, 7:00 am to 5:00 pm. CST.
Call Curium Pharmacovigilance / Product Monitoring Department at 866.789.2211
How is this product supplied
Order information
| Description | Qty | Unit | Size | Order # | NDC |
|---|---|---|---|---|---|
| Technescan HDP kit, 5 vial | 1 | Kit | 5 vial | N091B0 | 69945-091-20 |
| Technescan HDP kit, 30 vial | 1 | Kit | 30 vial | N091D0 | 69945-091-40 |